Nyquist MedTech provides comprehensive global clinical, regulatory, and medical device data across key markets, from large markets like the US to emerging markets like China. Our platform incorporates clinical trials contributing to approval success, FDA approval documents, product development roadmap, device genealogy revolution, Posticates for identifying innovators, and insights from adverse event data. With NyquistMed, you'll gain access to mission-critical insights to accelerate your time-to-market.
SCHEDULE A DEMO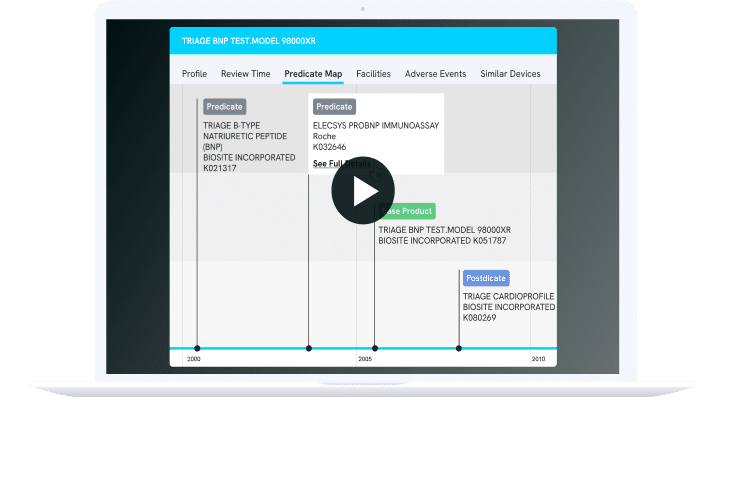
Get 20% more than the golden standard of clinicaltrial.gov by discovering trials that are outside the US in Japan, China, and more
Instantly uncover deeply-buried intelligence and break down the languages barriers by providing English translation for OUS
Access an AI-generated competitive landscape to help you save time finding the needle in the haystack
Enrich your global strategy by differentiated and detailed insights from the largest emerging markets such as China
Utilize AI-powered similar devices to help you identify unexplored sectors and indications
Set alerts to track changes in the pipeline, product, adverse events, and recalls so you never miss a catalyst event
Gain in-depth comprehensive insights across the world to make an informed regulatory strategy
Infuse fun into MAUDE with AI, which will summarize AE problems and aid in risk identification for your product
Utilize AI to make sense of all inspections and recalls, presenting you with visually engaging data to tell the story effectively
Get 20% more than the golden standard of clinicaltrial.gov by discovering trials that are outside the US in Japan, China, and more
Instantly uncover deeply-buried intelligence and break down the languages barriers by providing English translation for OUS
Access an AI-generated competitive landscape to help you save time finding the needle in the haystack
Enrich your global strategy by differentiated and detailed insights from the largest emerging markets such as China
Utilize AI-powered similar devices to help you identify unexplored sectors and indications
Set alerts to track changes in the pipeline, product, adverse events, and recalls so you never miss a catalyst event
Gain in-depth comprehensive insights across the world to make an informed regulatory strategy
Infuse fun into MAUDE with AI, which will summarize AE problems and aid in risk identification for your product
Utilize AI to make sense of all inspections and recalls, presenting you with visually engaging data to tell the story effectively
 U.S.
U.S. Australia
Australia Canada
Canada Japan
Japan EU
EU China
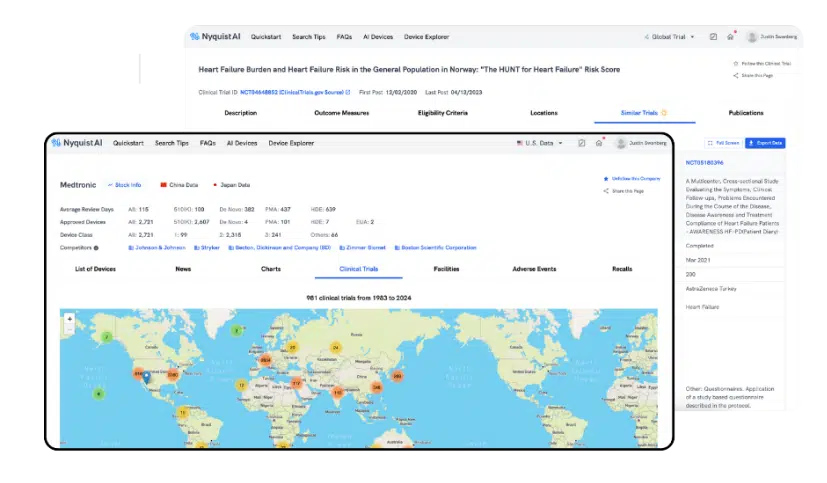
ChinaSave time and effort in global trial site research, collection, cleaning, and analysis with our Clinical Trial module. Unlike traditional databases, our AI comprehends context and connects information seamlessly. Forget multiple filters and use natural language search to find trials by indication, technology, patient type, and more.
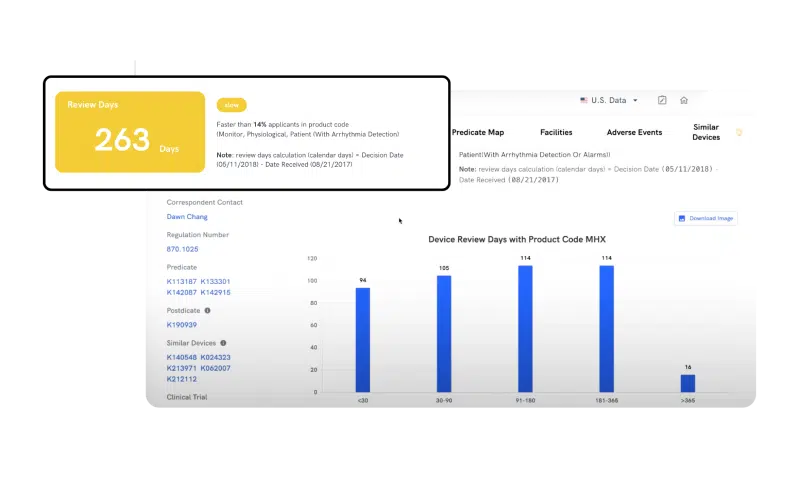
Remove uncertainty and effortlessly estimate clearance timelines for your devices. By leveraging the Review Time feature, you can now benchmark against peers and track timelines for similar devices in your product code, improving your regulatory planning process and saving precious time.
Discovering the ideal predicate is paramount for a successful 510(k) clearance. Nyquist MedTech empowers you to uncover predicates effortlessly, whether based on indication, technology, or natural language descriptions, mirroring how your engineering team conveys the product to regulatory authorities.
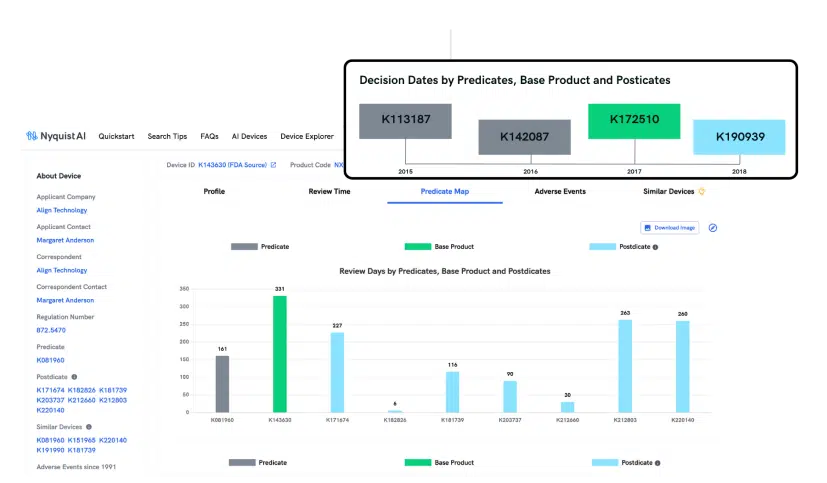
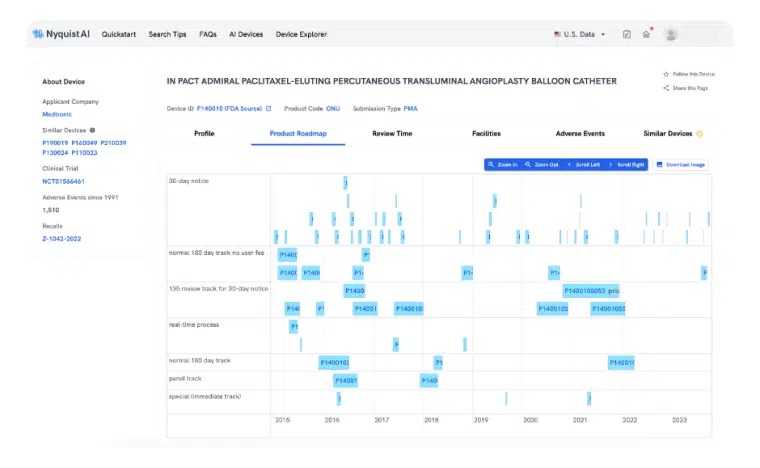
Preparing a PMA can be slow and cumbersome, but not with Product Roadmap. We simplify the collection, analysis, and extraction of precise insights throughout the entire product launch cycle. Gain a comprehensive view of how prior successes expand indications, modify labels, and improve design seamlessly.
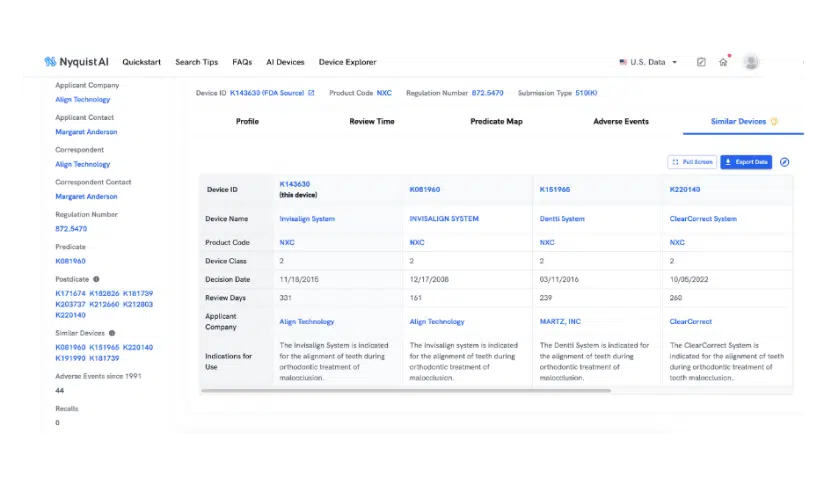
Our AI excels at contextual understanding and connecting the dots. Utilize Similar Devices to assess the competitive landscape and effortlessly discover devices from various sectors that address the same indications. With just one click, our AI instantly presents the top five similar devices based on your query.
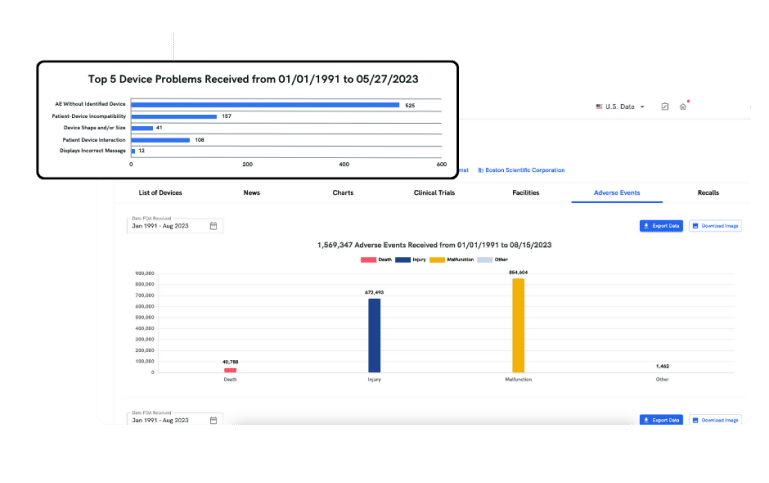
Browsing the MAUDE adverse event database can be time-consuming and manual. With adverse events, you gain quick access to essential safety data and critical insights, helping you detect significant risk signals, benchmark against peers, and identify trends from vast data.
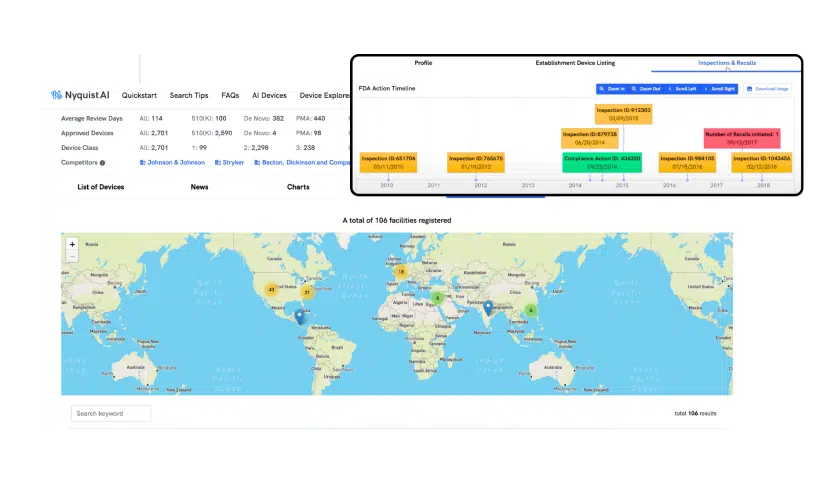
Quickly explore the registered OEM facilities available worldwide. Determine which is best for your device by reviewing product manufacturing history, inspections, citations, or recalls. You can also track your own facilities’ inspections and benchmark them against your competitors.
NyquistAI is a growing technology company providing an AI-powered solution to transform decades of clinical and regulatory data and documentation into actionable insights for Life Sciences professionals.
LEARN MORE